Work Packages
The CLAIMS project will develop, validate, and seek regulatory approval for a 𝗰𝗼𝗺𝗽𝗮𝗻𝗶𝗼𝗻 𝗱𝗶𝗮𝗴𝗻𝗼𝘀𝘁𝗶𝗰 𝗽𝗹𝗮𝘁𝗳𝗼𝗿𝗺 that provides a holistic view of each patient. This platform will visualize existing and new biomarker data, as well as predict disease trajectories under different treatment scenarios while accounting for comorbidities.
To achieve these goals, the project is divided into 7 Work Packages (WP). These WPs can be grouped into three main blocks, with WP’s interacting according to the links and dependencies described below:
WP1, 2 and 3 will work together to collect and harmonise retrospective data needed to develop the AI-driven prognostic models. They will ensure that the newly developed companion diagnostic platform allows the visualisation of all relevant data, including the outcomes of the AI-driven models. In the latter half of the project, we will validate these models and the companion diagnostic platform in a prospective study.
WP4 and 5 will focus on generating new biomarkers and tools to improve the accuracy and predictive value of our models developed in WP2.
In support of the project structure, WP6 will deal with communication, dissemination and exploitation activities, while WP7 will be dedicated to the overall coordination and management of the project.
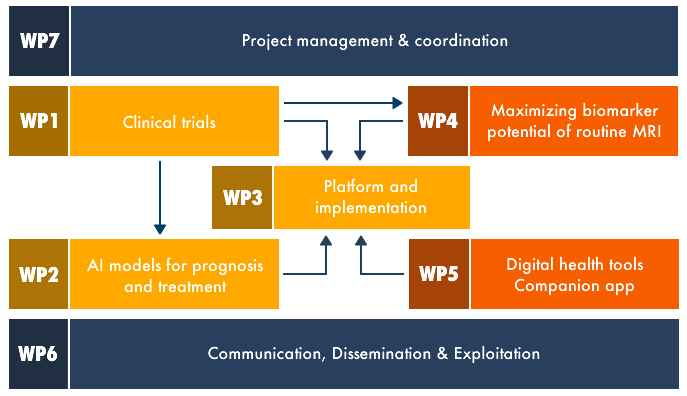
Work package 1
focuses on the coordination and management of the retrospective and prospective clinical studies.
Work package 2
aims to develop and validate AI-driven models for patient subtyping, prognosis and personalised treatment optimisation.
Work package 3
will implement a companion diagnostics platform which makes the innovations developed in the other work packages easily accessible for prospective clinical evaluation as well as for routine clinical care of MS patients.
Work package 4
aims to better disentangle Relapse Associated Worsening (RAW) from Progression Independent of Relapse Activity (PIRA) using MRI biomarkers. We will focus on taking the detection of PIRA out of the research stage and bringing it towards daily clinical care. Simultaneously, these biomarkers for PIRA will will feed into the prognostic models of WP2 once they become available.
Work package 5
will transfer the next-generation of smartphone-captured biomarkers from research setting towards daily clinical practice for MS care throughout Europe.
Work package 6
Communication, Dissemination & Exploitation
Work package 7
Project Management and Coordination









