Mission
To enable data-driven precision medicine for people with Multiple Sclerosis.
Vision
CLAIMS aims to revolutionize Multiple Sclerosis care by developing a data driven tool for treatment optimisation, one that improves long-term patient outcomes and reduces the economic burden on individuals and society.
Introduction
Multiple sclerosis (MS) is a devastating immune-mediated disorder of the central nervous system (CNS). Global prevalence of MS has increased from 2.1 million in 2008 over 2.8 million in 2020 and continues to rise to date (Walton et al., 2020). The classical view on MS describes different clinical subtypes, with relapsing-remitting MS (RRMS) being the most common form, occurring in 85% of patients (National MS Society). Patients with RRMS experience neurological exacerbation (relapses) as well as intermittent periods of remission in which they remain clinically stable. Relapses can either recover completely or leave persistent clinical disability. Among these patients, approximately two-thirds progress to secondary-progressive MS (SPMS) (Tutuncu et al., 2013). In contrast to RRMS, the disease course of patients with SPMS or primary-progressive MS (PPMS, 15% of MS patients) is mainly driven by a gradual worsening of disability in the absence of relapse activity (Lublin et al., 2014).
Recent research has challenged this classical view of distinct MS subtypes, as they may not sufficiently account for the large spectrum of multifaceted clinical phenotypes and disease courses as well as sub-clinical disease variability (Kuhlmann et al., 2023). The irreversible worsening of disability can occur at any stage of the disease and is driven mainly by 2 processes. First, people with MS (pwMS) may accumulate disability due to relapses, known as Relapse Associated Worsening (RAW, Figure 1.A), and second, pwMS may accumulate disability not associated with acute inflammatory relapses which is referred to as Progression Independent of Relapse Activity (PIRA, Figure 1.B). Recent studies on disease progression in MS have shown that PIRA gradually becomes the dominant driver of disability worsening as the disease progresses (Lublin et al., 2022). Additionally, it was shown that for some pwMS, PIRA occurs already during the very early phases of the disease, and this is associated with worse long-term outcomes (Tur et al., 2022).
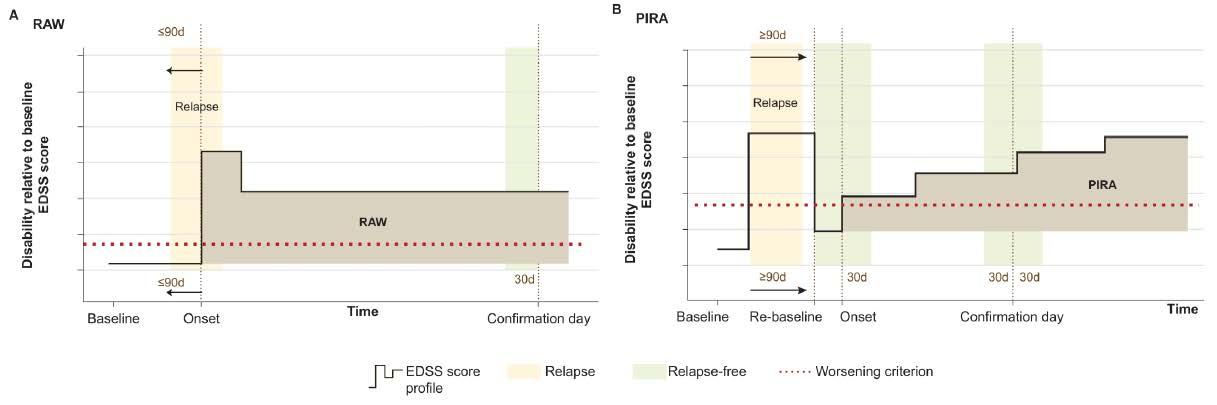
Although there are many Disease Modifying Treatments (DMTs) approved by the European Medicines Agency, most of these were developed to target acute inflammatory relapses, thus lesion formation. Therefore, and not surprisingly, clinical decision making and treatment optimisation is highly focussed on these acute inflammatory lesions. It was only in 2017 that the first DMT for treatment of progressive MS was regulatory approved in the US and Europe (Montalban et al.,2017), and nowadays more clinical trials aiming to treat progressive MS are on their way. The latter is driven by accumulating evidence that PIRA drives disability accumulation even in the absence of RAW (Lublin et al., 2022).
Objectives
The CLAIMS project aims to address the urgent need for a data-driven treatment optimisation tool for pwMS, one that addresses the new insights in disease worsening due to both relapses and disease progression independent of relapses, and as such supports optimal treatment decisions and improved long-term patient outcomes.
As such, the project will develop, validate, and seek regulatory approval for a companion platform that provides a holistic view of each patient. This platform will visualize existing and new biomarker data, as well as predict disease trajectories under different treatment scenarios while accounting for comorbidities. Powered by deep-learning-based disease subtyping and progression models, this platform aims to enhance the precision of MS care, extending the patients’ quality-adjusted life years, and reducing the economic burden on both individuals and society.
1
2
3
4
References:
- Kuhlmann et al, 2023 – doi:10.1016/S1474-4422(22)00289-7
- Lublin et al., 2014 – doi:10.1212/WNL.0000000000000560
- Lublin et al., 2022 – doi:10.1093/brain/awac016
- Montalban et al., 2017 – doi:10.1056/NEJMoa1606468
- Tur et al., 2022 – doi:10.1001/jamaneurol.2022.4655
- Tutuncu et al., 2013 -doi:10.1177/1352458512451510
- Walton et al., 2020 – doi:10.1177/1352458520970841









